Oxidative cleavage of the double bond of oleic acid to obtain nonanoic and azelaic acids
Summary of the work made
Azelaic acid is an important monomer for obtaining polyesters. We have studied different pathways for breaking and oxidizing the oleic acid double bond with the aim to obtain azelaic acid. This technology is still under development and we are looking for an industrial partner available to continue the research in collaboration with us.
Work
We have studied a process for the production of azelaic and pelargonic acid, by oxidative cleavage of the double bond of oleic acid in two reaction steps. In the first step, the monoenic reagent react with hydrogen peroxide in the presence of pertungstic acid, as catalyst, giving a diol, as reaction product, that can be easily separated and purified. The key factors influencing this reaction have been studied. The second step is the oxidative cleavage with molecular oxygen of the diol formed in the first step, in the presence of a catalyst formed in situ from tungstic acid and cobalt acetate. The nature of the catalyst probably a polyoxametallate, has been studied; factors affecting conversion and yields have been examined in detail.
The oxidative cleavage of the double bond with the formation of two carboxylic groups can be obtained, as mentioned, in two steps, In the first step, a well known reaction of double bond oxidation to vicinal diols is performed by using hydrogen peroxide in the presence of tungstic acid that is oxidized to pertungstic. This diol is an interesting by-product and it can be obtained also by following a different route, that is, by performing epoxidation of the double bond (see section on ESBO production) and successive hydrolysis.
In the second step, diols formed can further be oxidized with molecular oxygen, under mild pressures, in the presence of both tungstic acid, residual of the first reaction, and a cobalt salt, such as the acetate. The carbon-carbon bond of the diols, in this conditions, is broken giving two carboxylic groups with satisfactory yields and selectivities.
This two steps process is simple to realize and occurs in mild conditions, therefore, it can be considered as a new promising route to the synthesis of azelaic acid, alternative to the process commonly used in industry, based on the oxidative cleavage of the double bond via ozonolysis.
The first reaction step of hydroxylation occurring with the following stoichiometry:
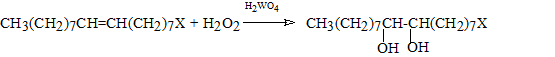
X= COOH, OH
has been studied by different authors. Starting from their observations we have tried to improve the yields of the reaction, to separate and purify the obtained diols. The second step of the reaction, that is, the oxidative cleavage of the diol primarily obtained:
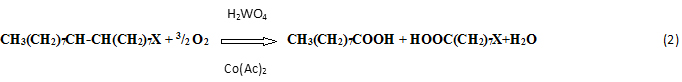
promoted by the presence of both Co(Ac)2 and H2WO4 has not been reported in any scientific paper, but only in an old patent literature [1]. Therefore, we studied in particular the less known second step reaction [2] with the aim: (i) to find the best operative conditions, (ii) to improve yields and selectivities , (iii) to understand the catalytic mechanism, (iiii) to recover efficiently the products from the reaction mixtures. The catalyst formed “in situ”, between the exhaust pertungstic acid, used in the first reaction step, and the added cobalt acetate, seems to have the property to involve molecular oxygen in an heterolytic process of oxidation. We have used oleic acid as reagent for the relevant industrial interest in producing azelaic acid but mainly oleyl alcohol because reagent and products are, in this case, easier to separate and analyse giving us the opportunity of a deeper investigation on both the reaction steps. This work has been performed in collaboration with
Novamont SpA.
-
G. Sabarino, A. Gardano, M. Foà, PCT/EP93/02944, WO 94/10122
-
E. Santacesaria, A. Sorrentino, F. Rainone, M. Di Serio, F. Speranza; “Oxidative cleavage of the double bond of monoenic fatty chains in two steps: a new promising route to azelaic acid and other industrial products”. Ind. Eng. Chem. Res. 39, 2766-2771 (2000) 