Dehydroclorination of chlorohydrins to epichlorohydrin
Summary of the work made
Di-chlorohydrins can be produced starting in alternative from propene or glycerol. In a successive step the dehydrochlorination of the di-chlorohydrins gives place to epichlorohydrin, a very important intermediate in the production of epoxy resins. We have studied this process at different levels from laboratory to the industrial plant. Epichlorohydrin is more volatile than di-chlorohydrin and must be removed, as soon as possible, from the reaction environment to avoid secondary reaction lowering the yield. For this reason, the reaction is performed in a distillation column. A suitable kinetic model has been developed to simulate the distillation column with reaction. Different industrial plants have been built following this technology.
Work
Epichlorohydrin is an important raw material for the production of some polymers such as epoxide resins, synthetic elastomers, and sizing agents for the papermaking industry. The process for the synthesis of epichlorohydrin is a rather old process. The classical process starts from propene and through a first step, consisting in a high temperature chlorination, allyl chloride is obtained; subsequently, allyl chloride with hypochlorous acid gives place to glycerol dichlorohydrins isomers and finally the reaction of the glycerol dichlorohydrins with sodium hydroxide or calcium hydroxide, leads to epichlorohydrin. The main reactions involved in the epichlorohydrin synthesis are shown in Figure 1. As it can be seen, from the second step of this process a mixture of dichlohydrins is obtained, more precisely the reaction product is a mixture composed of 70% of 1,2-DCH and 30% of 1,3-DCH. The reactivity of 1,2-DCH, the most abundant component, is much lower. This
requires an opportune reactor size (reactive distillation column) to obtain a satisfactory conversion of the less reactive reactant and gives place to the formation of undesired by-product as a consequence of the long residence time.
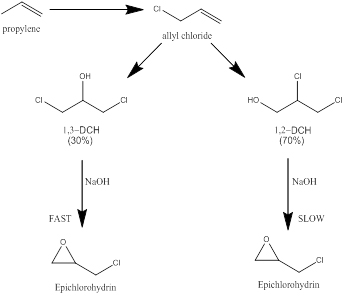
The availability of great amounts, at low cost, of glycerol stimulated the development of an alternative process based on the use of this substance as raw material instead of propene, as it can be seen in thesection devoted to “Glycerol as building block for new synthesis”. However, in this section, the successive step is described, that is the process of epichlorohydrin synthesis by chlorohydrins dehydrochlorination. Dehydrochlorination is normally performed in a reactive distillation column for stripping, as soon as possible, epichlorohydrin from the reaction environment to avoid undesired reaction paths lowering the yields. The reaction requires an alkaline catalyst that could be a solution of NaOH or an aqueous suspension of Ca(OH)2. In both cases, the main occurring reaction is the dehydrochlorination of 1,2 1nd 1,3 DCH to epichlorohydrin, but hydrochloric acid, formed as a consequence of the reaction, reacts with the catalyst neutralizing it. If Ca(OH)2 is
used as catalyst the pH of the solution does not change very much during the reaction, because its solubility in water is not very high and the formation of CaCl2 together with dissolved Ca(OH) gives place to a buffered solution. On the contrary, working with NaOH a dramatic change of pH occurs as a consequence of the reaction and NaOH must be continuously added to keep the pH of the reacting solution relatively constant. The reactor modeling requires a deep knowledge of the kinetic law of the occurring reactions and related parameters to reduce the amount of by-products (glycidol, glycerol, mono-chlorohydrin). All these aspects have been described in details by Carrà et al. in some papers published many years ago but still valid [1,3]. A similar approach is valid also for the production of propene oxide by dehydrochlorination of the corresponding monochlorohydrins [4,5].
Scheme of a distillation column with reaction for the production of epychlorohydrin
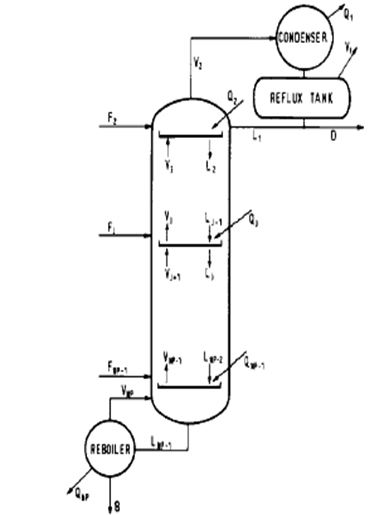
The production of epichlorohydrin by 1,2 and 1,3 DCH dehydrochlorination
-
S. Carrà , E. Santacesaria, M. Morbidelli, P. Schwarz, C. Divo; "Synthesis of epichlorohydrin by elimination of hydrogen chloride from chlorohydrins. 1. Kinetics aspects of the process". Ind. Eng. Chem. Proc. Des. Dev., 18, 424 (1979).

-
S, Carrà , E. Santacesaria, M. Morbidelli, P. Schwarz, C. Divo;"Synthesis of epichlorohydrin by elimination of hydrogen chloride from chlorohydrins. 2. Simulation of the reaction unit". Ind. Eng. Chem. Proc. Des. Dev., 18, 428 (1979).

-
S. Carrà , M.Morbidelli, E. Santacesaria, "Distillation problems in the epoxides synthesis through dehydrochlorination". Proceedings of the International Congress, Advances in Separation Science, Sept. 18-21, Trieste, Italy (1978).
-
S. Carrà , E. Santacesaria, M. Morbidelli, L. Cavalli; "Synthesis of propylene oxide from propylene chlorohydrins. Kinetic aspect of the process". Chemical Engineering Science, 34, 1123-1132 (1979)

-
S. Carrà , M.Morbidelli, E. Santacesaria, G. Buzzi "Synthesis of propylene oxide from propylene chlorohydrins. 2. Modeling of the distillation with chemical reaction unit". Chemical Engineering Science, 34, 113-1140 (1979).
