Propene epoxidation with TS-1
Summary of the work made
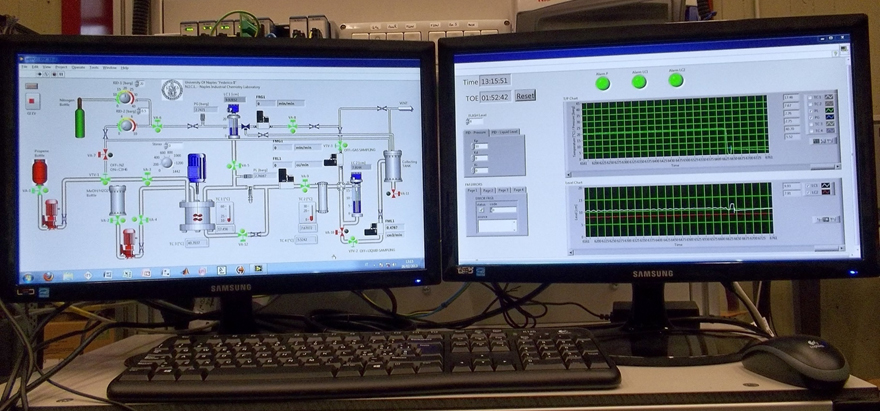
We have studied both the kinetics of all the reactions involved in the propene epoxidation with H2O2, in the presence of titanium silicalite (TS-1) as catalyst (main and side reactions), and mass transfer limitation. A completely automatized pilot plant for studying the reaction has been realized in which different types of continuous reactors (slurry or tubular) can be tested (see picture). This technology is still under development with our industrial partner.
Work
PropPropene oxide is a building block of the modern chemical industry. The research related to the development of convenient and greener processes has lead to the development of the HPPO (Hydrogen Peroxide Propene Oxide) process, where propene is oxidized by hydrogen peroxide in the presence of methanol as solvent and titanium silicalite (TS-1) as catalyst. Even if the first industrial plants based on this technology are already running, only few information have been published till now about this process on the current literature, in particular regarding the kinetics of th side reactions that lower the yields and increase the cost of propene oxide purification. It is well known that propene oxide (PO) is a highly reactive chemical used as an intermediate for the production of numerous commercial materials such as polyether polyols (polyglycol ethers), propene glycols and propene glycol ethers. Nowadays, several routes to produce PO are available and the emerging
one is the direct epoxidation of propene with hydrogen peroxide (HPPO). By using TS-1 catalyst, the reaction is normally carried out under mild conditions (40-50 °C and 20-30 bar), and theoretically, only water is generated as byproduct according to the following reaction:
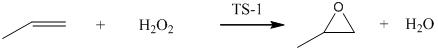
In 2008, after about 25 years from the first ENI patent on the subject, Evonik (former Degussa) and SKC have launched the first commercial-scale propene oxide plant, based on the HPPO technology, with a capacity of 100 ktons/year. Recently, BASF and DOW Chemical started with a new plant based on a similar technology, with a 300 ktons/year capacity and some new plants were put on stream or are under construction.
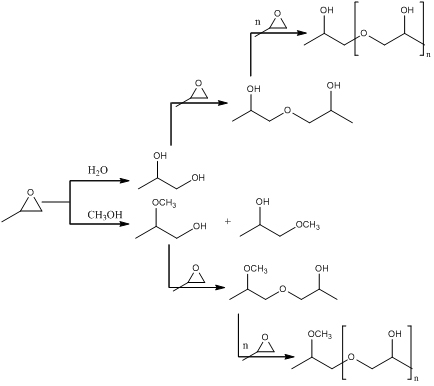
By considering the scientific literature on the subject, a lot of effort has been made by considering the catalyst synthesis and the investigation on the reaction mechanism but only three papers have been published till now concerning the HPPO kinetics. These papers are all mainly focused on the main reaction investigation. Moreover, two of the mentioned papers report kinetic runs performed by using isopropanol as solvent instead of methanol, that is, the solvent most commonly employed in the industrial plants. As before mentioned, the cited papers are focused only on the main reaction, giving no kinetic information related to the side-reactions that reduce the yield of the process and increase the costs of PO purification. As a matter of fact after the PO formation, PO can continue to react giving places to different by-products, as it can be seen in the following scheme:
Epoxide ring opening reactions
All the reactions reported in the scheme are related to the ring opening reactions, and are clearly responsible of lowering the PO selectivity. PO can react either with water or methanol, giving place to respectively propene glycol and 1 and 2-methoxy propanol. Then, the reaction proceeds, because PO reacts with either methoxy propanol or glycol, giving dimers that can react further forming different oligomerization products. It must be pointed out that the formation of oligomers is detrimental for the selectivity of the catalyst. In conclusion, the adsorption of hydrogen peroxide on TS-1 leads to the formation of titanium hydroperoxide acid sites that are responsible of the desired reaction but are also responsible of an increase in the ring opening reaction rate as it derives from the study of the kinetics of the ring opening reactions.
Another reaction important for this process is the hydrogen peroxide decomposition occurring in particular at high temperature:
Another reaction important for this process is the hydrogen peroxide decomposition occurring in particular at high temperature:

This reaction is important for two reasons: (i) the cost of hydrogen peroxide is one of the main parameter in the economy of the process, (ii) the hydrogen peroxide decomposition gives place to molecular oxygen that can lead to explosive mixtures with propene.
At last, there is also the possibility of methanol oxidation to formic acid, that then further react with methanol to give methyl formiate:


Methyl formiate has a boiling point similar to PO, therefore, its presence in the reaction mixture is a serious problem in PO purification.
The literature on the subject has recently been reviewed by us [1] and the kinetics of all the mentioned reactions (main and side reactions) have been studied in details [2] identifying for any reaction the corresponding kinetic law and the related kinetic parameters.
A completely automatized pilot plant for studying the reaction has been realized in which different types of continuous reactors (slurry or tubular) can be tested (see picture) [3].
-
V. Russo, R. Tesser, E. Santacesaria, M. Di Serio; Chemical and Technical Aspects of Propene Oxide Production via Hydrogen Peroxide (HPPO Process); Industrial & Engineering Chemistry Research (2013) 52, 1168-1178

-
V. Russo, R. Tesser, E. Santacesaria, and M. Di Serio; Kinetics of Propene Oxide Production via Hydrogen Peroxide with TS-1; Ind. Eng. Chem. Res. 2014, 53, 6274−6287

-
Pictures of the laboratory pilot plant for producing propene oxide with the HPPO technology developed by CONSER-NICL-EUROCHEM