Miscellanea
In this section other technologies studied by our group, not reported before, will be briefly listed. A brief comment on the topic is followed by the references to works and patents published on the subject.
1. Titanium dioxide preparation and post-treatments for increasing its stability
The hydrolysis of titanium (IV) sulfate solution is the most important step in the manufacture of TiO2 via sulfate process. We have studied the precipitation of titanium dioxide [1,2] interpreting the experimental results with a mathematical model containing three population balance equations, one related to the free particles, another to the agglomerates and the last to the total particles. In this model kinetic laws for respectively nucleation and growth have been introduced. The population balance equations have been solved by adopting the method of moments. We obtained as results: the amount of precipitated titania during the time, the average size of the elementary particles and of the aggregates along the time and the evolution with time of the corresponding distribution function. Different runs, performed at different temperatures, titanium concentration and sulfuric acid concentration, have been interpreted.
After the precipitation a relatively unstable
form of titania is obtained (anatase) by filtration. This needs to be transformed in a more stable form (rutile) by heating in an oven at high temperature. This process has been also studied for defining time and temperature for completing the transformation from anatase to rutile.
At last, titania must be coated for preventing the photochemical activity of its surface, before it can be used. We have developed a method to achieve a compact coating of different oxides, in particular alumina on the titania surface by reacting titanium dioxide with methal chloride in vapour phase [3-6].
Literature on Titanium dioxide preparation and post-treatments for increasing its stability
-
Santacesaria, M. Tonello, G. Storti, R. C. Pace, S. Carrà; "Kinetics of Titanium Dioxide Precipitation by Thermal Hydrolysis; J. of Colloid and Interface Sci. 111, 1,44 (1986).

-
E. Santacesaria, S. Carrà , G. Storti, R. C. Pace; "Kinetics of titanium dioxide precipitation by thermal hydrolysis". VIII CHISA, International Congress of Chemical Engineering, 3- 7/9/1984, Praga, Cecoslovacchia.
-
E. Santacesaria, S. Carrà , R.C. Pace, C. Scotti;"Vapor-phase treatment of titanium dioxide with metal chlorides. Note 2: Kinetics of the reaction between titanium dioxide and aluminium
chloride". Ind. Eng. Chem. Prod. Res. Dev., 21, 501 (1982).

-
E. Santacesaria, S. Carrà , R. C. Pace, C. Scotti; "Vapor-phase treatment of titanium dioxide with metal chlorides. Note 1: The reactions of coating performed by Al2Cl6, SiCl4 and ZrCl4, in the vapor phase".Ind. Eng. Chem. Prod. Res. Dev., 321, 496-500 (1982).

-
C. Scotti, R.C. Pace, S. Carrà , E. Santacesaria; "Process for the post-treatment of titanium oxide". (Montedison SpA) Eur. Pat. 799P42368
-
C. Scotti, R.C. Pace, S. Carrà , E. Santacesaria;"Post treated Titanium Dioxide, a process for obtaining the same, and a process for preparing a titanium dioxide pigment". (Montedison SpA) Eur. Pat. 799P41367; US Pat. 4,374,675
2. Process for the production of polyethylene therepthalate (PET)
The transesterification of dimethyl terephtalate (DMT) with ethylene glycol (EG) have been studied in batch conditions following along the time, in the presence of different catalysts, the evolution of all the possible oligomers [1,2].
This kinetic approach is useful for:
-
screening the most suitable catalyst;
-
modeling transesterification plants;
-
giving the initial conditions for the successive polymerization step.
For the scope of accelerating the catalysts screening for respectively transesterification and PET formation some mono-functional model molecules have been adopted, as reagents, that greatly simplify the analysis of the transesterification products [3,4].
At last, two new heterogeneous alkaline catalysts have been found (hydrotalcite, and MgO) highly promoting the transesterification reaction [5,6].
Literature on Process for the production of polyethylene therepthalate (PET)
-
E. Santacesaria, F. Trulli, L. Minervini, M. Di Serio, R. Tesser, S. Contessa;“Kinetic and Catalytic Aspects in Melt
Transesterification of Dimethyl Terephthalate With Ethylene Glycol” J. of Applied Polymer Science 54,9,1371-1384 (1994).

-
M. Di Serio, R. Tesser, F. Trulli, E. Santacesaria; “ Kinetic and Catalytic Aspects in Melt Transesterification of Dimethylterephthalate with Ethylene Glycol in the Presence of Different Catalytic Systems. Journal of Applied Polymer Science, 62,409 (1996).

-
B. Apicella, M. Di Serio, L. Fiocca, R. Po, E. Santacesaria; “Kinetic and catalytic aspects of the formation of poly(ethyleneterephtalate) (PET) investigated with model molecules” J. of Applied Polymer Science 69, 2423-2433 (1998).

-
M. Di Serio, B. Apicella, G. Grieco, P. Iengo, L. Fiocca, R. Po,
E. Santacesaria; “Kinetic and catalytic aspects of dimethylterephtalate transesterification also through the use of model molecules”. J. of Molecular Catalysis. A: Chemical 130, 233-240 (1998).

-
Di Serio, M.; Tesser, R.; Ferrara, A.; Santacesaria, E.; “Heterogeneous basic catalysts for the transesterification and the polycondensation reactions in PET production from DMT.” Journal of Molecular Catalysis A: Chemical (2004), 212(1-2), 251-257.

-
E. Santacesaria, M. Di Serio, R. Tesser, A. Ferrara; Processo per la produzione di polialchilen-tereftalati. Brevetto Italiano ITNA2003A000034. 2003
3. Methanol steam reforming
Methanol plays a fundamental role both as a building block for the production of many chemicals such as, for example, formaldehyde and acetic acid, or as a low-cost energy vector like MTBE, biodiesel and gasoline production, this last on H-ZSM5 based catalysts (MTG Mobil process). Moreover, methanol has also been considered a hydrogen reservoir that allows us to overcome the problems related to hydrogen storage and transportation. The production of hydrogen, for example, as a combustible in fuel cell applications, can be readily carried out through methanol catalysed steam reforming. The most active and selective catalysts for this endothermic reaction have been found to be commercial CuO-ZnO-Al2O3 based catalysts, developed for the low temperature CO gas shift reaction. Methanol steam reforming (MSR) can give good yields of hydrogen through the following reaction:
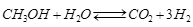
We studied the kinetics of this reaction by using two different types of reactors,that is, a well stirred continuous reactor [1] and a tubular reactor [2]. Moreover, the comparison of the different kinetic models proposed in the literature and different catalyst has also been made [3].
Literature on Methanol steam reforming
-
E. Santacesaria, S. Carrà; "Kinetics of catalytic steam reforming of methanol in a CSTR reactor". Applied Catalysis 5, 345-358 (1983).

-
Santacesaria E., R.Tesser, M. Di Serio “Kinetics of Methanol Steam Reforming on Cuo-ZnO catalysts.” Chemical Engineering Transaction; Vol. 4, 191-197, (2004)
-
Tesser, R.; Di Serio, M.; Santacesaria, E.; Methanol steam reforming: A comparison of different kinetics in the simulation of a packed bed reactor”. (2009); Chemical Engineering Journal (2009), 154(1-3), 69-75.

4. Relevant properties of new fluorocarbons compounds
The well known and undesired ozone depletion property of chlorofluorocarbons (CFC) is the main reason for their progressive elimination from the market and is the basis for the need of suitable alternatives. As a replacement for these substances, in the field of aerosol propellants, of foam blowing agents and mainly of refrigerant fluids, the attention has been focused on the hydrofluorocarbons (HFC) and their mixtures. There is a growing need, for these compounds, to collect and correlate experimental data regarding phase equilibria in order to asses the range of utilization and to validate suitable thermodynamic models for data interpretation. We have made an exhaustive and uniform approach to the problem of phase equilibrium description for binary system constituted by a volatile refrigerant (HFC) and a polymer-like lubricant oil [1,2]. Also the use of CFC as blowing agent has been considered [6] and a polyurethane foam formation has been modeled
[6].
Literature on Relevant properties of new fluorocarbons compounds
-
R.Tesser, M. Di Serio, R.Gargiulo, G. Basile, L. Bragante, E. Santacesaria; Vapour-liquid equilibrium measurements for binary mixtures of R32, R143a, R134a and R125 with a perfluoropolyether lubricant. Journal of Fluorine Chemistry 121(2003),1, 15-22.

-
Tesser, Riccardo; Passaretti, Adele; Di Serio, Martino; Bragante, Letanzio; Santacesaria,E; “Phase Equilibria in Binary Mixtures Refrigerant + Fluorinated Lubricating Oil: Vapor-Liquid and Liquid-Liquid Measurements.” Journal of Chemical and Engineering Data (2004), 49(4), 838-846.

-
E. Musso, R. Tesser, G. Basile, M. Di Serio, E. Santacesaria;“Description of liquid-liquid equilibrium in binary and multicomponent mixtures of CFC/Lubrificanting oil by means of an extented Flory-Huggins Model”. J. of Fluorine Chem. 103, 41-51 (2000).

-
Musso, M. Di Serio, R. Tesser, L. Cavallo, G. Basile, E. Santacesaria; “ Vapour pressures of fluorocarbons in polyols, polyamines and polycarboxyls”; Journal of Fluorine Chemistry 78 (1996) 167-175.

-
G. Basile, E. Musso, R. Tesser, M. Di Serio, E. Santacesaria;“Polyol and Blowing Agent Binary System Described by Means of the Flory Model” Cellular Polymers 13,98-112 (1994)
-
G. Basile, E. Musso, R. Tesser, M. Di Serio, E. Santacesaria; “Polyol and Blowing Agent Binary System Described by Means of the Flory Model”; Cellular Polymers 13,98-112 (1994)
-
Tesser, R.; Di Serio, M.; Sclafani, A.; Santacesaria, E.;“Modeling of polyurethane foam formation” Journal of Applied Polymer Science (2004), 92(3), 1875-1886.

5. Skeletal isomerization of 1-butene to isobutene
A new catalyst obtained by grafting silicon alkoxide on the surface of alumina have shown high activity and selectivity in the skeletal isomerization of 1-butene to isobutene [1]. The prepared catalyst has a moderate acidity and resulted much more active than silica-alumina and alumina catalysts. Moreover, it resulted much more resistant to poisoning than the two other mentioned catalysts.
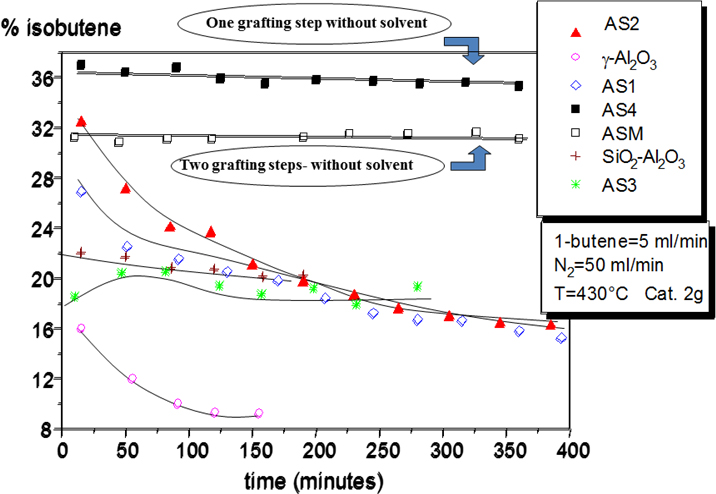
Literature on Skeletal isomerization of 1-butene to isobutene
-
Santacesaria, E.; Cozzolino, M.; Tesser, R.; Di Serio; “Skeletal isomerization of 1-butene to isobutene over acid catalysts obtained by grafting silicon alkoxide on -alumina.” M. DGMK Tagungsbericht (2004), 2004-3 (Proceedings of the DGMK-Conference "C4/C5-Hydrocarbons: Routes to Higher Value-Added Products", 2004), 77-85.